BIO-PROTOCOL, vol. 14, 1361, 2024
Abstract
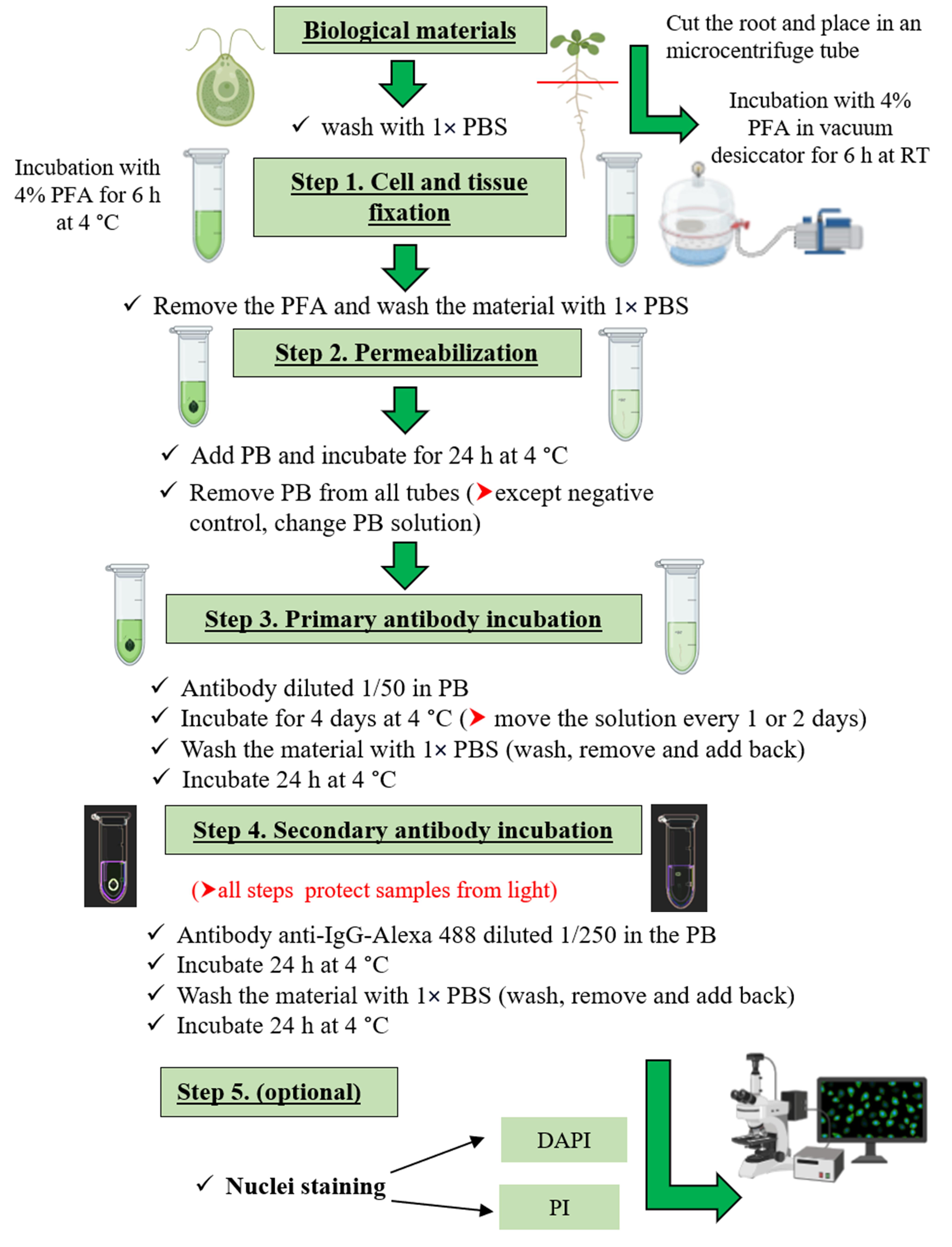
The target of rapamycin (TOR) is a central hub kinase that promotes growth and development in all eukaryote cells. TOR induces protein synthesis through the phosphorylation of the S6 kinase (S6K), which, in turn, phosphorylates ribosomal S6 protein (RPS6) increasing this anabolic process. Therefore, S6K and RPS6 phosphorylation are generally used as readouts of TOR activity. Protein phosphorylation levels are measured by a western blot (WB) technique using an antibody against one specific phosphosite in cell extracts. However, at the tissue/cell-specific level, there is a huge gap in plants due to the lack of alternative techniques for the evaluation of TOR activity as there are for other organisms such as mammals. Here, we describe an in vivo protocol to detect S6K phosphorylation in tissues/cells of model photosynthetic organisms such as Arabidopsis thaliana and Chlamydomonas reinhardtii. Our proposed method consists of the immunolocalization of a phosphorylated target of TOR kinase using a fluorescent secondary antibody by confocal microscopy. The protocol involves four main steps: tissue/cell fixation, permeabilization, and incubation with primary and secondary antibodies. It is an easy technique that allows handling different samples at the same time. In addition, different ultrastructural cell markers can also be used, such as for nucleus and cell wall detection, allowing a detailed analysis of cell morphology. To our knowledge, this is the first protocol to detect TOR activity in situ in photosynthetic organisms; we consider that it will pave the research on the TOR kinase, opening new possibilities to better understand its complex signaling.
Key features
• The protocol is an easy and non-destructive method to detect S6K phosphorylation at the cellular level for plants and algae.
• First method for in situ immunolocalization of target proteins of TOR kinase in photosynthetic organisms.